Continuing Review of an Approved and Ongoing Protocol Irb Graduate Center
The IRB administrator will conduct a pre-review of the continuing review request for an IRB protocol that is set to expire. The process may involve requesting initial modifications from the PI. Once the continuing review request is ready for a committee member review, the assigned IRB administrator will assign the protocol with a due date to specific reviewers. You will receive an email and a task indicating you are assigned a protocol to review. You can either following the link in the email or login to RASS and go to your task list. Note: If you are unable to complete the assigned review, contact irbhp@cornell.edu ASAP to let them know and the protocol will be re-assigned to another reviewer.
- When you log into RASS, go to your tasks.
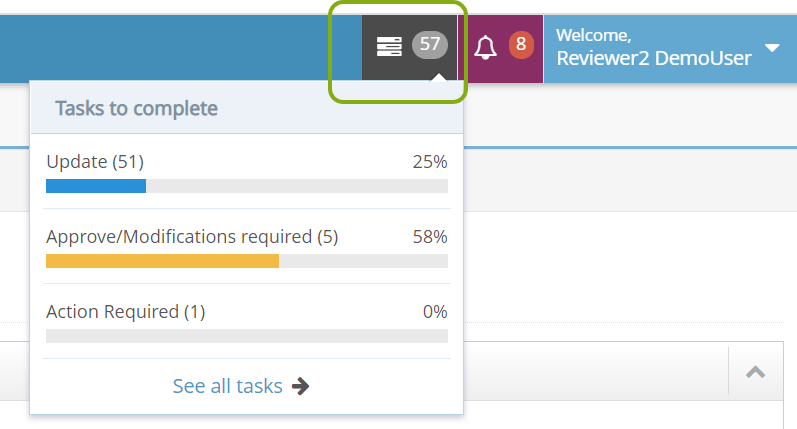
- Locate the tasks that indicate an IRB protocol is ready for review. Note the Stage and Due Date of the review. Click the Message text of the task to open the IRB Protocol.

- Once you are in the IRB protocol you need to review, the first thing you will see is an Approval Form. Don't do anything with this form yet. This will be your last step upon completion of your review.
- Note: key information about the protocol such as PI, Status and Review Type can be found in the header at the top of the page.
- You may want to click Expand All to open all the sections of the protocol.
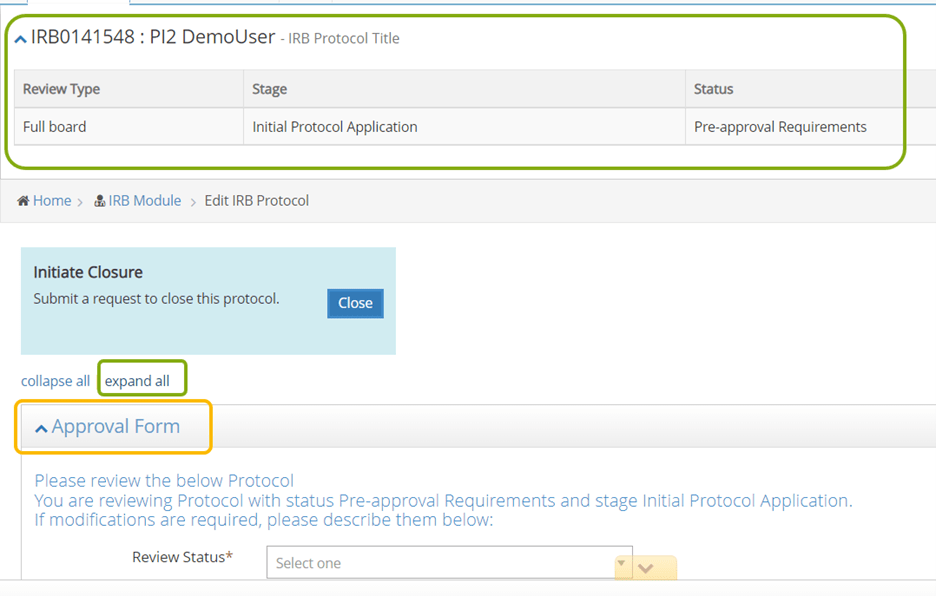
- Using the panel shortcuts on the left side of the screen, you can skip around to different sections in the protocol. This may be useful in finding key information needed for your review.
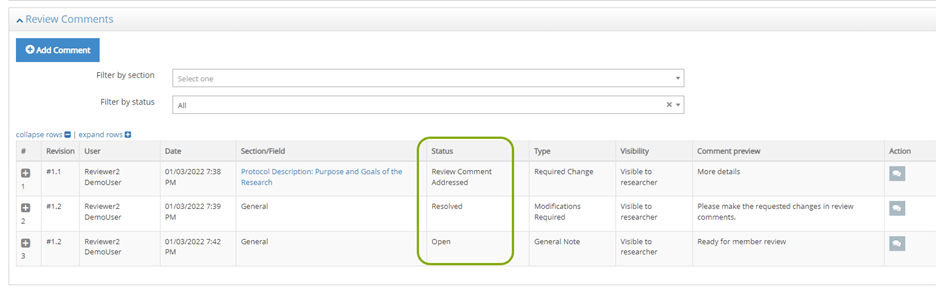
- Click on Review Comments to read the comments entered by the IRB Administrator or other IRB Reviewers. The IRB Administrator may include specific notes or recommendations for the review in this section.
- Pro Tip: For continuing reviews and amendments, it is recommended that you review the changes made to the protocol during this submission. To do this go to the Requirements section.
- Click on Primary Info to find the assigned IRB Administrator.
- Click on Continuing Review to see the continuing review form. The PI or research team provided the requested information for the continuing review in this form. NOTE: If this submission was for only a continuing review (no amendment), this will be the only section of the protocol form in which changes occurred.
- Click on Review Type Determination to see information about risk level, special participant populations, and the presumed review level (e.g., Exempt, Expedited, Full Board). The system auto-determines a review level, but the IRB Administrator can choose to override this determination.
- Click on Additional Documents to see any relevant documentation provided by the research team. Note: For protocols imported from the previous system, all prior approval letters are in Other Documents and are labeled IRB-Protocol_protocol#_v#.pdf. These do not need review.
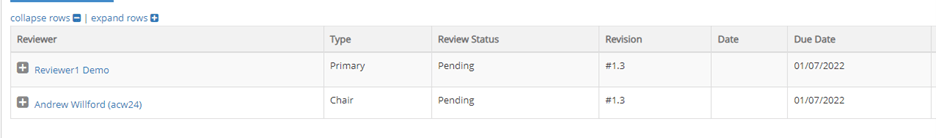
- Click on Reviewers to see if any other reviewers are also assigned to review the protocol or to find the due date for your review.
Review Comments
- Review the protocol by reviewing the information in the continuing review section and each of the protocol sections if the protocol was also amended. Scroll down the protocol or use the panel shortcuts to go to a specific section of the protocol. Note that some attached documents will be located within a specific section (i.e., the informed consent form will be in the panel titled Informed Consent), while others may be in the panel titled Additional Documents (e.g., survey instruments, letters of support, SOPs).
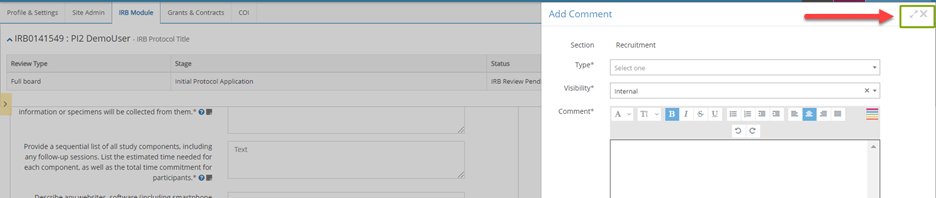
- To add your own review comments, click on the icon that looks like a note at the field or section level.
- When adding a comment, first select the type of comment (e.g., required change, suggested change, general note).
- Add your comment in the corresponding text box. Choose Save.
- Your review comment will be added to the table found in the Review Comments section (near the top of the protocol). Your comments are marked as Internal so that they are only visible to the IRB Administrator and other reviewers, not visible to the PI or Research Team.
- You can edit or delete your own comments using the action buttons.
- Add Comment within the Review Comments panel allows you to add general comments about the protocol.
Pro-Tip: Use the split screen button when adding review comments to review the protocol on the left side of the screen while adding comments in the Add Comment window on the right side of the screen.
Approval Form
- Once you have finished your review, scroll or use the panel shortcuts to go to the Approval form near the top of the protocol.
- Select a Review Status.
- Choose Modifications Needed for Admin Review if you do not need to re-review the protocol after the PI makes the requested changes, and you instead want the assigned IRB administrator to finalize the protocol review and approval at that point in time.
- Choose Modifications Needed for Member Review if you would like to re-review the protocol after the PI makes the requested changes.
- Choose Modifications Needed for Convened Review if this is a Full Board review and you think the protocol will be ready to go directly to the IRB Committee Meeting once the PI has made your requested changes (without your re-review).
- Choose Referred to Convened meeting if this is a Full Board review and changes are not needed prior to a discussion of the protocol at an IRB committee meeting.
- Choose Approved if this is an Expedited review and you approve the protocol without any changes needed. Note: choosing this Review Status will not automatically shift the protocol into an Approved status; an IRB Administrator will need to finalize the documentation first.
- Add your notes in the Details Note: You will not see the Details section if you choose Approved.
- Choose Submit. The assigned IRB Administrator will be notified that your review is complete and will work with the PI on modifications needed if indicated as part of your review. If you indicated the protocol can be approved, the IRB Administrator will finalize that documentation and alert the PI.

- If you requested to re-review a protocol following changes, you will receive an email and task to complete your re-review once the PI has responded. Follow these same steps to complete your re-review.
- Pro-Tip: Review only the most recent changes made by the PI by comparing revisions. Scroll down or use the panel shortcuts to go to the Requirements section. Choose your revisions and click Compare.
Source: https://guide.rass.cornell.edu/institutional-review-board-for-human-participant-research/irb-committee-member-review-of-a-protocol-requesting-continuing-review/
0 Response to "Continuing Review of an Approved and Ongoing Protocol Irb Graduate Center"
Post a Comment